Development and application of biomedical titanium metal materials
The development of biomedical titanium and titanium alloys can be divided into three generations. The first generation is mainly pure titanium and T-6AI-4V alloy, and the second generation is a+β type titanium alloy typified by Ti-5AI-2. 5Fe and T-6AI-7Nb. The third generation is the development and development of titanium alloys with better biocompatibility and lower modulus of elasticity. The low elastic modulus β-type titanium alloy without Al.V is the main research direction. Pure titanium and T-6AI-4V are the earliest applications of titanium and titanium alloys. Pure titanium has the advantages of non-toxicity, light weight, good corrosion resistance and good biocompatibility. In the early 1940s, Bothe first introduced pure titanium to the biomedical field. In the 1960s, Branemark used pure titanium for oral cavity. The implant has made rapid development and application of pure titanium as a medical implant material. Due to the low strength of pure titanium, the wear resistance is poor, which limits its application in large load-bearing structural parts. Currently, it is mainly used for bearing capacity. Bone replacement for smaller parts (eg, oral prosthetics, etc.). T-6AI-4V (TC4) titanium alloy is the earliest and most widely used titanium alloy. It has high strength and good processing performance. It was widely used in clinical practice in the late 1970s to make hip joints and knee joints. Such as surgical repair and replacement materials. However, with the development of biomedicine, a large number of experiments and data have confirmed that V has toxic side effects on the human body, and even exceeds Cr and Ni, which can cause metabolic disorders and induce malignant tissue reactions.
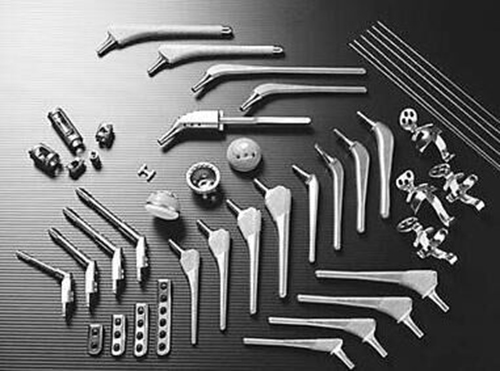
Medical field and orthopedic field of titanium alloy
Market conditions With the development of titanium alloys, the increase in the number of titanium materials and the reduction in prices, the use of titanium in the civil industry has multiplied. CFDA divides medical devices into three grades according to their safety from high to low, and is supervised and managed by three levels of government. Titanium and titanium alloy implants belong to the third category of medical devices and are high-value consumables. . Sub-sectors with more than 5% of market segments include in vitro diagnostics, cardiac, imaging diagnostics, orthopedics, ophthalmology, and plastic surgery. Among them, in vitro diagnosis, orthopedics and cardiac intervention are the fastest high-value consumables in China. The application of biomedical titanium and its alloy materials has gone through three signature stages [27]: in the early 1950s, first in the United Kingdom and the United States, commercial pure titanium was used to make bone plates, screws, intramedullary nails and hip joints. . The Swiss company Mathys also uses Ti-6A1-7Nb alloy to make non-reamed interlocking intramedullary nail system (including humerus, tibia, femur) and hollow screws for the treatment of femoral neck fracture. Porous Ni-Ti (PNT) alloy bioactive materials for the manufacture of cervical and lumbar interbody cages (Cage) Canada's BIORTHEX Corporation has developed a porous Ni-Ti alloy patented material ACTIPORE to manufacture cervical and lumbar interbody fusion cages for the treatment of orthopedic spinal injuries. The new beta titanium alloy can take into account the orthopedics, dental and vascular interventions and other advanced materials orthopedic medical device industry accounted for 9% of the global medical device market share, and is still in rapid growth. The orthopaedic medical device market is divided into four areas of trauma, joints, spine and others. Among them, the trauma category is the only segment that has not been occupied by foreign companies in the main market share. The main reason is that the products in this field are low in technical content, easy to imitate, and the operation is difficult. Many second- and third-level hospitals can carry out, and foreign companies cannot fully cover them. . Trauma products can be divided into internal fixation and external fixation equipment. Internal fixation trauma products include intramedullary nails, bone plates and screws. In 2012, the domestic orthopedic market accounted for 34% of trauma, 28% of joints, 20% of spine, and others. 18%. Large joints belong to high-end medical devices, and technical barriers are high. Currently, mainstream hospitals mainly use imported materials for orthopedic materials. In terms of technology, design, research and development, materials, surface treatment, etc., there are still gaps between domestic and imported products. . Artificial joints are mainly divided into artificial knees, hips, elbows, shoulders, fingers, toe joints, etc. The most important joint replacements include hip joints and knee joints, which together account for more than 95% of the global joint replacement market. Spinal implant devices include thoracolumbar vertebral plate system, cervical plate system and cage system. The interbody fusion system is mainly used for the treatment of intervertebral disc replacement. It is also the most important subdivision field, accounting for the entire spine implant market. Half of it.
Email: jasontitanium@163.com
Whatsapp/Skype/Wechat/Line: +86-15829508781


 Titanium and Titanium alloy
Titanium and Titanium alloy
